What’s Laser?
A Laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. This term originated as an acronym for “Light Amplification by Stimulated Emission of Radiation“. Lasers produce a very narrow beam of light.
A laser differs from other sources of light in that it emits light which is coherent. Spatial coherence allows a laser to be focused to a tight spot, enabling applications such as laser cutting and lithography. Spatial coherence also allows a beam to stay narrow over great distances (collimation), enabling applications such as pointers and lidar. Lasers can also have high temporal coherence, which allows them to emit light with a very narrow spectrum, i.e., they can emit a single color of light. Alternatively, temporal coherence can be used to produce pulses of light with a broad spectrum but durations as short as a femtosecond (“ultrashort pulses”).
Lasers are used in optical disk drives, printers, barcode scanners, DNA sequencing instruments, fiber-optic, semiconducting chip manufacturing (photolithography), and free-space optical communication, surgery and skin treatments, cutting and welding materials, military and law enforcement devices for marking targets and measuring range and speed, and in laser lighting displays for entertainment. They have been used for car headlamps on luxury cars, by using a blue light and a phosphor to produce highly directional white light.
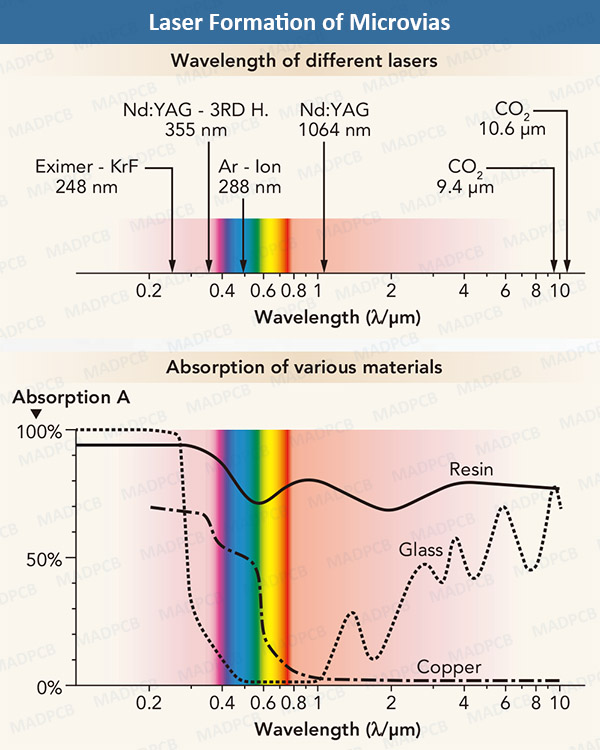
Laser technology is widely applied for printed circuit board (PCB) and flexible printed circuit (FPC) manufacturing now, can be used to make PCB profile, cut coverlay, EMI film and PSA, and ablating microvia in HDI board. The figure below is a example of laser application in HDI PCB’s micovia (μVia) formation.
in PCB industry, laser machines refer to copper ablation, dielectric ablation, or copper-and-dielectric ablation and flexible material cutting. Choosing a PCB manufacturer, which is equipped with these machines, helps improving your board quality and the lead time of your products to market.
Absorbing Energy
Although more modern views of the atom do not depict discrete orbits for the electrons, it can be useful to think of these orbits as the different energy levels of the atom. In other words, if we apply some heat to an atom, we might expect that some of the electrons in the lower-energy orbitals would transition to higher-energy orbitals farther away from the nucleus.
Once an electron moves to a higher-energy orbit, it eventually wants to return to the ground state. When it does, it releases its energy as a photon — a particle of light. You see atoms releasing energy as photons all the time. For example, when the heating element in a toaster turns bright red, the red color is caused by atoms, excited by heat, releasing red photons. When you see a picture on a TV screen, what you are seeing is phosphor atoms, excited by high-speed electrons, emitting different colors of light. Anything that produces light – fluorescent lights, gas lanterns, incandescent bulbs — does it through the action of electrons changing orbits and releasing photons.
The Laser/Atom Connection
A laser is a device that controls the way that energized atoms release photons. “Laser” is an acronym for light amplification by stimulated emission of radiation, which describes very succinctly how a laser works.
Although there are many types of lasers, all have certain essential features. In a laser, the lasing medium is “pumped” to get the atoms into an excited state. Typically, very intense flashes of light or electrical discharges pump the lasing medium and create a large collection of excited-state atoms (atoms with higher-energy electrons). It is necessary to have a large collection of atoms in the excited state for the laser to work efficiently. In general, the atoms are excited to a level that is two or three levels above the ground state. This increases the degree of population inversion. The population inversion is the number of atoms in the excited state versus the number in ground state.
Once the lasing medium is pumped, it contains a collection of atoms with some electrons sitting in excited levels. The excited electrons have energies greater than the more relaxed electrons. Just as the electron absorbed some amount of energy to reach this excited level, it can also release this energy. As the figure below illustrates, the electron can simply relax, and in turn rid itself of some energy. This emitted energy comes in the form of photons (light energy). The photon emitted has a very specific wavelength (color) that depends on the state of the electron’s energy when the photon is released. Two identical atoms with electrons in identical states will release photons with identical wavelengths.
Laser Light
Laser light is very different from normal and has the following properties:
- The light released is monochromatic. It contains one specific wavelength of light (one specific color). The wavelength of light is determined by the amount of energy released when the electron drops to a lower orbit.
- The light released is coherent. It is “organized” — each photon moves in step with the others. This means that all of the photons have wave fronts that launch in unison.
- The light is very directional. A laser light has a very tight beam and is very strong and concentrated. A flashlight, on the other hand, releases light in many directions, and the light is very weak and diffuse.
To make these three properties occur takes something called stimulated emission. This does not occur in your ordinary flashlight — in a flashlight, all of the atoms release their photons randomly. In stimulated emission, photon emission is organized.
The photon that any atom releases, has a certain wavelength that is dependent on the energy difference between the excited state and the ground state. If this photon (possessing a certain energy and phase) should encounter another atom that has an electron in the same excited state, stimulated emission can occur. The first photon can stimulate or induce atomic emission such that the subsequent emitted photon (from the second atom) vibrates with the same frequency and direction as the incoming photon.
The other key to a laser is a pair of mirrors, one at each end of the lasing medium. Photons, with a very specific wavelength and phase, reflect off the mirrors to travel back and forth through the lasing medium. In the process, they stimulate other electrons to make the downward energy jump and can cause the emission of more photons of the same wavelength and phase. A cascade effect occurs, and soon we have propagated many, many photons of the same wavelength and phase. The mirror at one end of the laser is “half-silvered,” meaning it reflects some light and lets some light through. The light that makes it through is the laser light.
Types of Lasers
There are many different types of lasers. The laser medium can be a solid, gas, liquid or semiconductor. Lasers are commonly designated by the type of lasing material employed:
- Solid-state lasers have lasing material distributed in a solid matrix (such as the ruby or neodymium:yttrium-aluminum garnet “Yag” lasers). The neodymium-Yag laser emits infrared light at 1,064 nanometers (nm). A nanometer is 1×10-9 meters.
- Gas lasers (helium and helium-neon, HeNe, are the most common gas lasers) have a primary output of visible red light. CO2 lasers emit energy in the far-infrared, and are used for cutting hard materials.
- Excimer lasers (the name is derived from the terms excited and dimers) use reactive gases, such as chlorine and fluorine, mixed with inert gases such as argon, krypton or xenon. When electrically stimulated, a pseudo molecule (dimer) is produced. When lased, the dimer produces light in the ultraviolet range.
- Dye lasers use complex organic dyes in liquid solution or suspension as lasing media. They are tunable over a broad range of wavelengths.
- Semiconductor lasers, sometimes called diode lasers, are not solid-state lasers. These electronic devices are generally very small and use low power. They may be built into larger arrays, such as the writing source in some laser printers or CD players.
Laser Wavelengths
A ruby laser is a solid-state laser and emits at a wavelength of 694 nm. Other lasing mediums can be selected based on the desired emission wavelength (see table below), power needed, and pulse duration. Some lasers are very powerful, such as the CO2 laser, which can cut through steel. The reason that the CO2 laser is so dangerous is because it emits laser light in the infrared and microwave region of the spectrum. Infrared radiation is heat, and this laser basically melts through whatever it is focused upon.
Other lasers, such as diode lasers, are very weak and are used in today’s pocket laser pointers. These lasers typically emit a red beam of light that has a wavelength between 630 nm and 680 nm. Lasers are utilized in industry and research to do many things, including using intense laser light to excite other molecules to observe what happens to them.
Here are some typical lasers and their emission wavelengths:
- Argon fluoride (UV): 193nm
- Krypton fluoride (UV): 248nm
- Xenon chloride (UV): 308nm
- Nitrogen (UV): 337nm
- Argon (blue): 488nm
- Argon (green): 514nm
- Helium neon (green): 543nm
- Helium neon (red) 633nm
- Rhodamine 6G dye (tunable): 570-650nm
- Ruby (CrAIO3) (red): 694nm
- Nd:Yag (NIR): 1064nm
- Carbon dioxide (FIR): 10600
Laser Classifications
Lasers are classified into four broad areas depending on the potential for causing biological damage. When you see a laser, it should be labeled with one of these four class designations:
- Class I – These lasers cannot emit laser radiation at known hazard levels.
- Class I.A. – This is a special designation that applies only to lasers that are “not intended for viewing,” such as a supermarket laser scanner. The upper power limit of Class I.A. is 4.0mW.
- Class II – These are low-power visible lasers that emit above Class I levels but at a radiant power not above 1mW. The concept is that the human aversion reaction to bright light will protect a person.
- Class IIIA – These are intermediate-power lasers (cw: 1-5mW), which are hazardous only for intrabeam viewing. Most pen-like pointing lasers are in this class.
- Class IIIB – These are moderate-power lasers.
- Class IV – These are high-power lasers (cw: 500mW, pulsed: 10 J/cm2 or the diffuse reflection limit), which are hazardous to view under any condition (directly or diffusely scattered), and are a potential fire hazard and a skin hazard. Significant controls are required of Class IV laser facilities.